MRX006 Brochure
To download our brochure for MRX006 please enter your email address.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
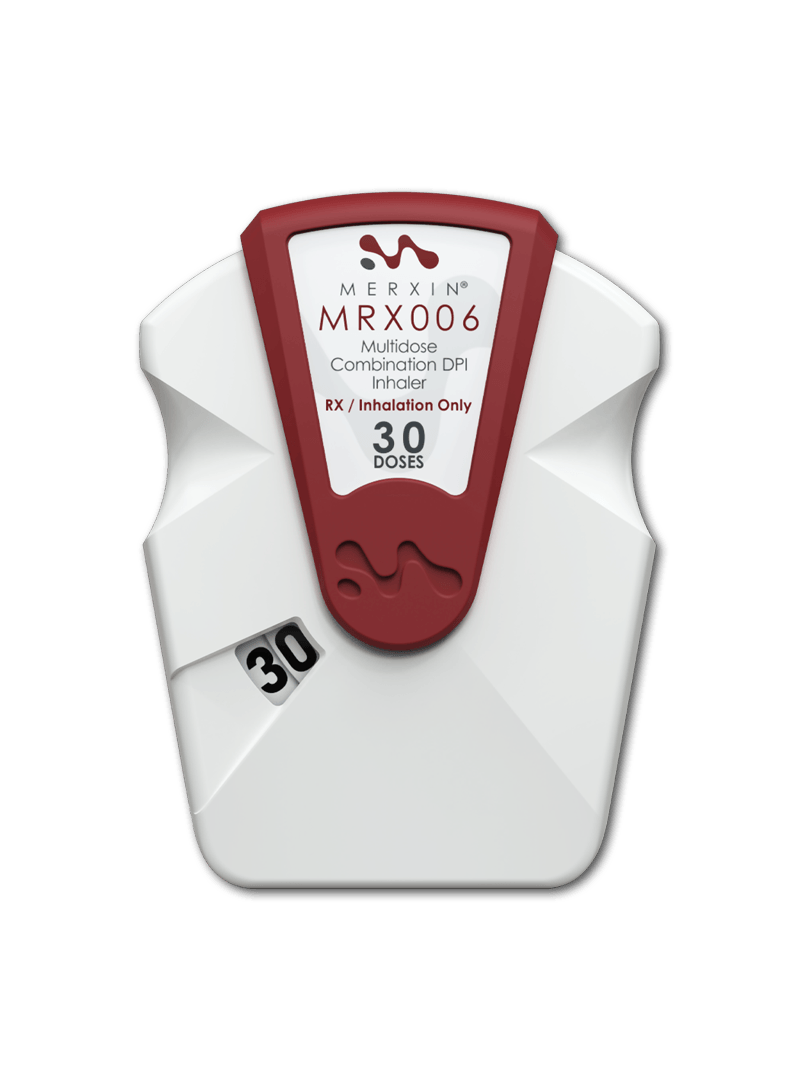
Dual cavity for triple therapy dry powder inhaler (DPI). MRX006 is based on the open-inhale-close principle. It is suitable as an AB-rated substitutable device for triple combination therapies.
It is designed to accommodate a combination formulation of 2 or more APIs, one of which may be chemically incompatible with the others, as with fluticasone, umeclidinium and vilanterol.
MRX006 offers unequalled technology tailored to current regulations and high-performance expectations.
Interested parties are invited to contact Merxin Ltd as soon as possible to secure access to a technology that will drive the next wave of inhaled finished dosage forms.
To download our brochure for MRX006 please enter your email address.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
MRX006 uses Merxin Ltd’s proprietary designs and does not knowingly infringe any IP.